Under the regulations that will be implemented soon, it will be a requirement of the A.N.M.A.T. that laboratories that own medicinal specialties that contain among their active ingredients pharmaceutical ingredients categorized as intermediate risk according to Provision No. 3185/99 carry out In Vivo or In Vitro Equivalence studies, as appropriate, in accordance with the provisions by Provisions (ANMAT) No. 3185/99, No. 5330/97, No. 5040/06, No. 17406/07 and No. 758/09.
FP Clinical Pharma - with its specialists in Biopharmaceutical, Bioavailability and Bioequivalence - makes advice and support available to colleagues in the Pharmaceutical Industry to respond to the demands of the new regulations. We offer the development of strategic plans and monitoring for the presentation of bioexemption protocols and results. It consists of a comprehensive service adapted and flexible to the characteristics of each product and the needs of each laboratory:
- Evaluation and Development of Strategies for Requesting Bioexemptions.
- Preparation of Bioexemption Protocols.
- Management and Monitoring of Permeability Tests.
- Management and Monitoring of Solubility Tests.
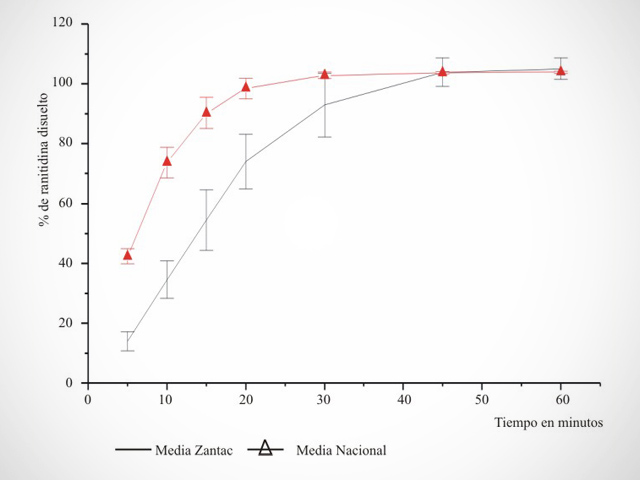
- Management and Monitoring of Dissolution Kinetics Studies.
- Pharmacokinetics Support for Definition of Permeability.
- Analysis of APIs- Excipients- In Vivo Absorption (PK) Interactions.
- Computational Simulations of In Vivo Trials.
- Support Bibliography for Bioexemptions.
- Scientific Writing for Support of Bioexemptions.
- Preparation of Results Dossier.
- In Vitro/In Vivo Relationship Analysis.
- Risk analysis.