We offer a wide range of services to support the development of its products before and during its marketing

Clinical Studies - Phase I Studies
We conduct Phase I to IV Clinical Research studies with the highest quality standards. A team made up of Medical Specialists from our research units together with the team of Medical Researchers form a group in charge of developing the protocols.
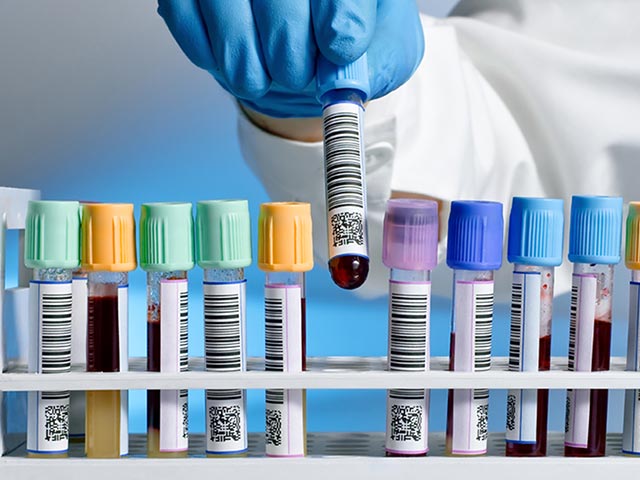
Bioavailability-Bioequivalence Studies
We are leaders of design, execution, and analysis of Bioavailability-bioequivalence and pharmacokinetic studies in Argentina
LATAM Pharmacovigilance
The team at FP CLINICAL PHARMA can help your company in developing a GOOD PHARMACOVILIGANCE PRACTICES PROGRAM according to your specific needs. THE GOOD PHARMACOVILIGANCE PRACTICES PROGRAM was designed as a quality system based on national and international guidelines and includes the investigation, detection, assessment, validation, monitoring, reporting and archiving of adverse events of the products during commercialization.
Monitoring and Coordination Services of Clinical Studies
We provide services for Phase 1, 2, 3 and 4 Studies. We have a team with vast experience in conducting clinical studies.

Bioanalytic Services
Cuantification of active principles and / or metabolites in biological samples for pharmacokinetic studies, bioavailability, bioequivalence, and phase I.
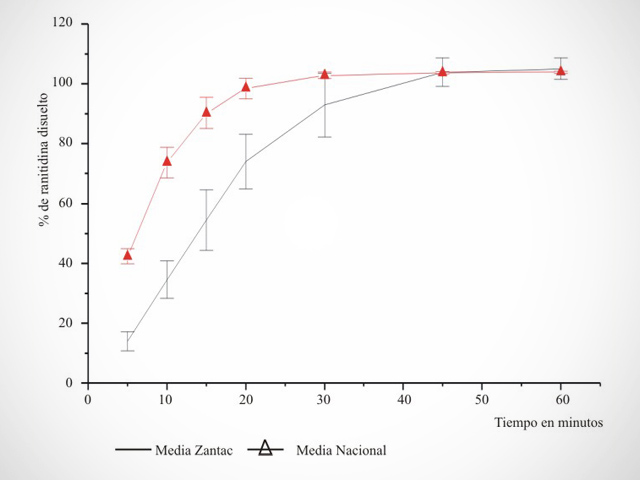
Bio-exemptions
Under the regulations that will be implemented soon, it will be a requirement of the A.N.M.A.T. that laboratories that own medicinal specialties that contain among their active ingredients pharmaceutical ingredients categorized as intermediate risk according to Provision No. 3185/99 carry out In Vivo or In Vitro Equivalence studies, as appropriate, in accordance with the provisions by Provisions (ANMAT) No. 3185/99, No. 5330/97, No. 5040/06, No. 17406/07 and No. 758/09.

Rational Medical
Writing Medical-Scientific Reports to support and endorse products on the market aimed at Regulatory Entities, Doctors, and much more.

